OUR PROGRAMMES
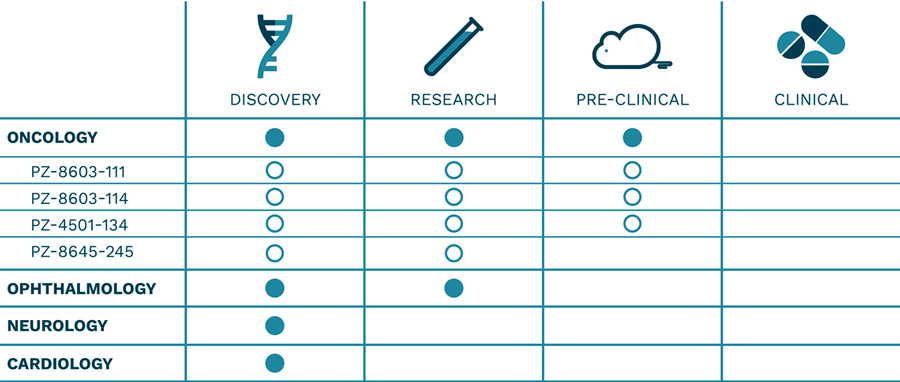
PIPELINE
Phyzat is focused in the discovery and development of innovative therapies addressing high-unmet medical needs, primarily in oncology, ophthalmology, neurology and cardiology Phyzat’s pipeline is intended to drive value directly through the clinical development process and to provide proof of concept for the platform technologies. Our internal preclinical and clinical development programs are designed to offer novel therapies through our proprietary drug candidates.
Clinical views
ONCOLOGY
Liver metastasis
Secondary liver cancer is a frequent advanced form of cancer that spread from another place in the body. Secondary liver cancer may be detected at the same time as the original cancer, when this is in an advanced phase; it may be detected in the course of treatment of the original cancer or even after successful treatment of the original cancer. Metastatic tumours limit the treatment options of the original tumour while accelerating the collapse of the organism.
The most common sites of primary tumours metastatic to the liver are colorectal, lung and breast cancer, which are also three of the four most frequent cancers in Europe and the most lethal. This program addresses primarily colorectal cancer (CRC) metastatic to the liver, although later on the same drug shall be tried against the original CRC tumour and against other cancers metastatic to the liver, including lung and breast cancer.

Colorectal cancer (CRC)
CRC is among the most commonly diagnosed cancers worldwide. It is a main concern of European Public Health (1). In 2012, according to GLOBOCAN estimates, there were 1.360.602 new cases of CRC worldwide (3rd most common cancer worldwide, accounting for 9,7% of all cancers apart from non‐melanoma skin cancers), and 447.136 new cases of CRC in Europe (2ndmost common cancer in Europe, accounting for 13,0% of all cancers apart from non‐melanoma skin cancers). In 2012, there were an estimated 693.933 deaths from CRC worldwide (8,5% of the total number of cancer deaths and 4th most common cause of cancer related deaths), of which 214.866 deaths from CRC in Europe (12,2% of the total number of cancer deaths and 2nd most common cause of cancer related deaths) (2). The 5‐year prevalence of CRC in 2012 was estimated at 3.543.582 worldwide and 1.203.943 in Europe. Approximately 25% of patients present with metastases at initial diagnosis and almost 50% of patients with CRC will develop metastases, contributing to the high mortality rates reported for CRC (3). In fact, approximately 60 to 70 percent of people with colorectal cancer eventually develop a liver tumor”. The 1‐ and 5‐year survival rates were 34,8% and 3,3% for synchronous liver metastases and 37,6% and 6,1% for metachronous liver metastases (4).Today, the median overall survival (OS) for patients with metastatic CRC being treated both in phase III trials and in large observational series or registries is∼30 months and more than double that of 20 years ago (5).
